Abstract
Background: In the AETHERA trial, brentuximab vedotin (BV) maintenance after autologous stem cell transplant (ASCT) in patients (pts) with high-risk relapsed/refractory classical Hodgkin lymphoma (r/r cHL) demonstrated an improvement in 2-year progression free survival (PFS) over placebo [Moskowitz 2015]. However, in clinical practice, pts are rarely able to complete all 16 cycles of BV at full dose due to toxicity. The aim of this retrospective, multicenter study was to investigate the effect of the cumulative maintenance BV dose on 2-year PFS in pts with high-risk r/r cHL. In an earlier analysis of this study, we found that 50-75% of the total cumulative BV dose attains a similar PFS advantage as >75% dose [Wagner 2022], we now report further findings with additional pts and a modified statistical analysis where total cumulative dose was treated as a time varying exposure.
Methods: Individual patient data were collected from those who received at least one cycle of BV maintenance after ASCT from 13 institutions across the United States. Pts were required to have at least one high-risk disease feature as defined in AETHERA: primary refractory disease (PRD), extra-nodal disease (END), or relapse < 12 months from the end of frontline therapy (RL <12). Pts were split in to 3 different cohorts based on total cumulative dose of BV maintenance. Cohort 1 (C1) included those who received > 75% of the planned total cumulative dose, cohort 2 (C2) included those who received 51 - 75%, and cohort 3 (C3) included those who received ≤ 50%. In order to avoid bias from conditioning on future exposure, the total cumulative dose was treated as a time varying exposure. We linearly interpolated when each dose was administered based on when the first and last dose of BV maintenance were administered. Pts were excluded if we did not have a date of first or last BV exposure. Adverse events were defined using CTCAE v. 5 criteria. A multivariate analysis was performed assessing the effect of total cumulative BV dose, RL <12, PRD, achieving less than a CR prior to ASCT, receiving two or more salvage therapies and prior BV exposure on 2-year PFS. Descriptive statistics were utilized, and survival outcomes were estimated using Kaplan-Meier.
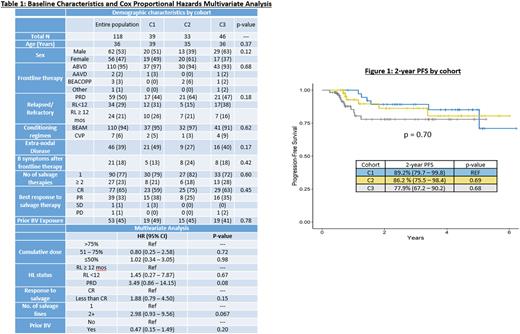
Results: A total of 123 pts were screened for inclusion; 5 did not have documented dates of first or last BV administration and were excluded, for a total of 118 evaluable pts. The median age was 36 (27 - 42) years, 53% being male, and the majority (95%) receiving ABVD as frontline therapy. High risk features were as follows: 50% of pts had PRD, 29% had RL<12 and the remaining 21% relapsed ≥ 12 months after initial therapy with 39% of total pts having END. Twenty-three percent had more than one line of salvage before ASCT. The majority of pts achieved a CR (65%) prior to ASCT and half (49%) had BV exposure prior to ASCT (majority during salvage) (Table 1).
The median follow up for the entire cohort was 2.96 years. The median number of BV maintenance cycles completed was 12 with the majority (69/118 = 59%) of pts discontinuing BV maintenance prior to completing all 16 cycles. Reasons for early discontinuation were as follows: 45 for toxicity, 10 for progression, 8 for patient preference, 2 for cost and 4 for other reasons. Forty-eight pts (41%) had a dose-reduction prior to discontinuation or completion of maintenance. Only 14% of pts (16/118) received the full cumulative dose of BV. One patient continued past 16 cycles and received a total of 25 cycles. Grade ≥3 adverse events for neuropathy, neutropenia, and infections were 17%, 7%, and 4% respectively.
The 2-year PFS was 89.2% for C1, 86.2% for C2, and 77.9% for C3 (p = 0.70, Figure 1). No variables were significant in the multivariate analysis for PFS (Table 1). Conclusions: The 2-year PFS was robust regardless of cumulative dose of BV maintenance. The 2-year PFS was superior for all groups compared to the original AETHERA trial, which had a 2-year PFS of 65% [Moskowitz 2015]. This difference could be due to higher rates of CR achieved with novel salvage agents prior to ASCT in this population versus the pre-ASCT CR reported in the AETHERA trial (37-38%) [Moskowitz 2015]. PFS was not affected by prior BV exposure during salvage therapy. These data are reassuring for pts who require dose reductions or discontinuation to manage toxicity and suggest that the PFS advantage may still be attained.
Disclosures
Wagner:Abbvie Inc.: Other: Partner is currently employed as a Medical Science Liaison . Kamdar:TG Therapeutics: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; AbbVie: Consultancy; AstraZeneca: Consultancy; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy; ADC Therapeutics: Consultancy; Beigene: Consultancy; ImpactBio: Consultancy; Seagen: Speakers Bureau. Torka:Targeted Oncology, Physician Education Review: Honoraria; Lilly USA: Consultancy; Epizyme: Consultancy; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Genentech: Consultancy. Modi:Seagen Inc.: Membership on an entity's Board of Directors or advisory committees; MorphoSys: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Karyopharm Therapeutics: Research Funding; Genentech: Research Funding; AstraZeneca: Honoraria; Beigene: Speakers Bureau. Ayyappan:AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Total CME: Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees. Brem:Morphosys/Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics/Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BeiGene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy; SeaGen: Speakers Bureau; KiTE Pharma: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees. Stephens:Novartis: Research Funding; CSL Behring: Consultancy; Celgene: Consultancy; AbbVie: Consultancy; AstraZeneca: Consultancy; Arqule: Research Funding; Acerta: Research Funding; Lilly: Consultancy; Genentech: Consultancy; TG Therapeutics: Consultancy; JUNO: Research Funding; Karyopharm: Research Funding; Mingsight: Research Funding; Beigene: Consultancy; Epizyme: Consultancy; Newave: Research Funding. Hu:ADC Therapeutics: Consultancy; Bristol Meyers Squibb: Consultancy; Caribou Biosciences: Research Funding; Celgene: Research Funding; CRISPR Therapeutics: Research Funding; Genentech: Research Funding; Lymphoma Research Foundation: Research Funding; Morphosys AG: Research Funding; Novartis: Consultancy; Repare Therapeutics: Research Funding. Shah:ADCT: Research Funding; BeiGene: Research Funding; Astrazeneca: Research Funding; Seattle Genetics: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; Epizyme: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.